CORMEDIX INC. REPORTS FOURTH QUARTER AND FULL YEAR 2023 FINANCIAL RESULTS AND PROVIDES BUSINESS UPDATE
Conference Call Scheduled for Today at 8:30 a.m. Eastern Time
Berkeley Heights, NJ – March 12, 2024 – CorMedix Inc. (Nasdaq: CRMD), a biopharmaceutical company focused on developing and commercializing therapeutic products for the prevention and treatment of life-threatening diseases and conditions, today announced financial results for the fourth quarter and full year ended December 31, 2023 and provided an update on its business.
Recent Corporate Highlights:
- On November 15, 2023, CorMedix received FDA approval for its new drug application (NDA) for DefenCath® (taurolidine and heparin). DefenCath is a catheter lock solution indicated to reduce the incidence of catheter-related bloodstream infections (CRBSIs) for the limited population of adult patients with kidney failure receiving chronic hemodialysis (HD) through a central venous catheter (CVC).
- The Company received an outpatient reimbursement determination for DefenCath from the Center for Medicare & Medicaid Services (CMS), which confirmed that DefenCath is eligible to receive a Transitional Drug Add-on Payment, or TDAPA, under the End Stage Renal Disease Prospective Payment System (ESRD PPS). CorMedix’s TDAPA application remains under review, and CMS has confirmed that it is working toward a July 1st effective implementation for TDAPA. Pending a timely implementation of TDAPA, CorMedix intends to launch in the outpatient setting in July 2024.
- CorMedix has intensified preparations for commercial launch, has staffed and trained experienced field sales and medical affairs organizations, and remains on schedule to begin commercialization in the inpatient setting on April 15, 2024.
- The Company is in the process of ramping up inventory production to meet anticipated demand, and remains on track to file with the FDA a supplement to the DefenCath NDA adding an alternate manufacturing site for finished dosage in April.
- CorMedix is targeting by the end of the first quarter to submit a post-approval meeting request to FDA with the objective of aligning with the agency on a clinical pathway for an expanded label at a proposed mid-year meeting.
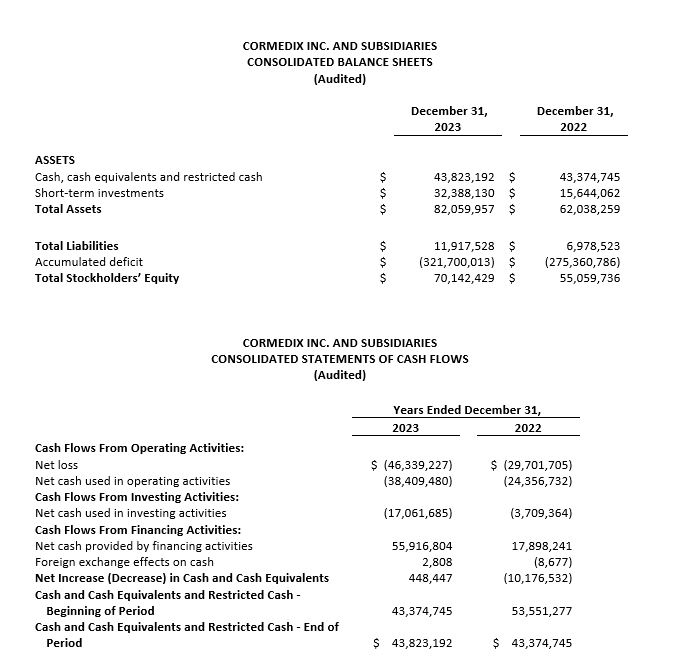
- Cash and short-term investments, excluding restricted cash, at December 31, 2023 amounted to $76.0 million.
Joe Todisco, CorMedix CEO, commented, “I am excited about the Company’s recent progress as we have scaled up activity ahead of our commercial launch in April. We have received significant inbound interest from both inpatient facilities as well as outpatient dialysis providers with respect to DefenCath, and we are actively engaged in customer discussions in both settings of care. I remain optimistic about the commercial potential for DefenCath, and the product’s ability to have a meaningful impact on CRBSI rates across the continuum of care in hemodialysis patients with CVCs.”
4th Quarter and Full Year 2023 Financial Highlights
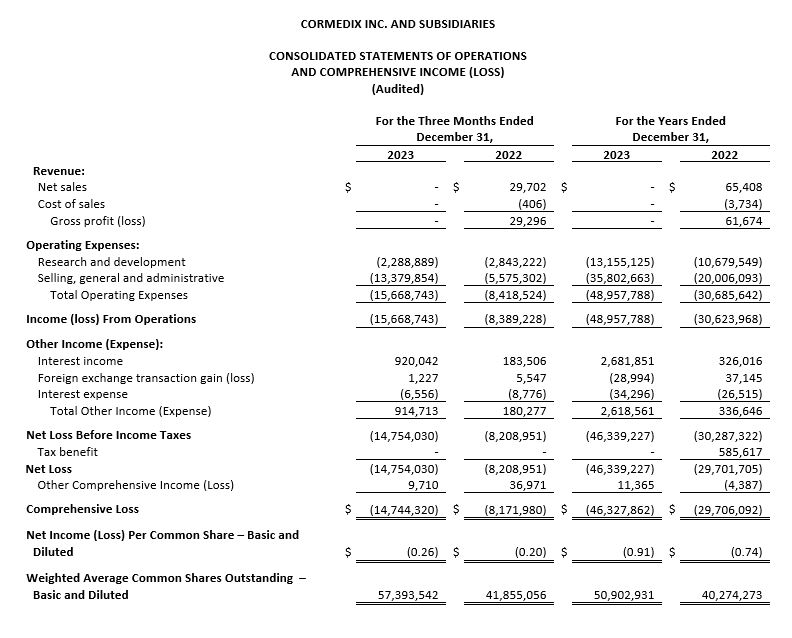
For the fourth quarter of 2023, CorMedix recorded a net loss of $14.8 million, or $0.26 per share, compared with a net loss of $8.2 million, or $0.20 per share, in the fourth quarter of 2022. The increase in net loss in the fourth quarter of 2023 compared with 2022 was primarily driven by increases in costs related to market research studies and pre-launch activities for DefenCath and increases in personnel expenses due to new hires in 2023 compared to the same period in 2022. Operating expenses during the fourth quarter of 2023 were $15.7 million, compared with $8.4 million in the fourth quarter of 2022, an increase of approximately $7.3 million.
For the year ended December 31, 2023, CorMedix recorded a net loss of $46.3 million, or $0.91 per share, compared with a net loss during the year ended December 31, 2022 of $29.7 million, or $0.74 per share. The increase in net loss was driven primarily by increases in operating expenses, primarily due to increased pre-launch commercial activities for DefenCath.
Operating expenses during the year ended December 31, 2023 amounted to $49.0 million compared with $30.7 million during the comparable period in 2022, an increase of $18.3 million, or 60%, due to a 79% increase and 23% increase in SG&A expense and R&D expense, respectively.
Total cash on hand, cash equivalents and short-term investments as of December 31, 2023 amounted to $76.0 million, excluding restricted cash of $0.2 million. The Company believes that it has sufficient resources to fund operations for at least twelve months from the issuance of its Annual Report on Form 10-K.
Conference Call Information
The management team of CorMedix will host a conference call and webcast today, March 12, 2023, at 8:30 AM Eastern Time, to discuss recent corporate developments and financial results. Call details and dial-in information is as follows:
Tuesday, March 12th @ 8:30am ET
Domestic: 1-888-886-7786
International: 1-416-764-8658
Conference ID: 08695074
Webcast: Webcast Link
DefenCath® (taurolidine and heparin)
IMPORTANT SAFETY INFORMATION
This brief summary does not include all the information needed to use DefenCath safely and effectively. Please see the full Prescribing Information for more information.
LIMITED POPULATION: DefenCath is indicated to reduce the incidence of catheter-related bloodstream infections (CRBSI) in adult patients with kidney failure receiving chronic hemodialysis (HD) through a central venous catheter (CVC). This drug is indicated for use in a limited and specific population of patients.
DefenCath is contraindicated and has warnings and precautions in patients with:
- Known heparin-induced thrombocytopenia (HIT).
- Known hypersensitivity to any drug products in DefenCath, including taurolidine, heparin or the citrate excipient or pork products.
If exposure to either of the above occurs, discontinue use of DefenCath and institute appropriate supportive measures.
The most frequently reported adverse reactions occurring in ≥2% of patients using DefenCath as a CLS were hemodialysis catheter malfunction, hemorrhage/bleeding, nausea, vomiting, dizziness, musculoskeletal chest pain, and thrombocytopenia.
To report any safety concerns including suspected adverse reactions, contact CorMedix Inc. at 1-888-424-6345 or FDA at 1-800-FDA-1088 or visit www.fda.gov/medwatch.
About CorMedix
CorMedix Inc. is a biopharmaceutical company focused on developing and commercializing therapeutic products for the prevention and treatment of life-threatening conditions and diseases. The Company is focused on commercializing its lead product DefenCath®, a non-antibiotic, antimicrobial catheter lock solution approved to reduce the incidence of catheter-related bloodstream infections in the limited population of adult patients with kidney failure receiving chronic hemodialysis through a central venous catheter. DefenCath was approved by the FDA on November 15, 2023. CorMedix anticipates the commercial launch of DefenCath in inpatient settings in April 2024 and in outpatient settings in July 2024, pending a timely implementation of TDAPA. CorMedix also intends to develop DefenCath as a catheter lock solution for use in other patient populations. For more information visit: www.cormedix.com.
Forward-Looking Statements
This press release contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, that are subject to risks and uncertainties. Forward-looking statements are often identified by the use of words such as, but not limited to, “anticipate,” “believe,” “can,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “will,” “plan,” “project,” “seek,” “should,” “target,” “will,” “would,” and similar expressions or variations intended to identify forward-looking statements. All statements, other than statements of historical facts, regarding management’s expectations, beliefs, goals, plans or CorMedix’s prospects, including, but not limited to, statements regarding the commercial launch of DefenCath, the timing of availability of DefenCath for inpatient and outpatient settings, DefenCath receipt of TDAPA, CMS implementation of TDAPA in July 2024, the interest in DefenCath by health systems, the ability to manufacture sufficient DefenCath for commercial launch, CorMedix’s future financial position, financing plans, future revenues, projected costs and the sufficiency of our cash and short-term investments to fund our operations, including the commercial launch of DefenCath, should be considered forward-looking statements. Readers are cautioned that actual results may differ materially from projections or estimates due to a variety of important factors, and readers are directed to the Risk Factors identified in CorMedix’s filings with the SEC, including its Annual Report on Form 10-K and its Quarterly Reports on Form 10-Q, copies of which are available free of charge at the SEC’s website at www.sec.gov or upon request from CorMedix. CorMedix may not actually achieve the goals or plans described in its forward-looking statements, and such forward-looking statements speak only as of the date of this press release. Investors should not place undue reliance on these statements. CorMedix assumes no obligation and does not intend to update these forward-looking statements, except as required by law.
Investor Contact:
Dan Ferry
Managing Director
LifeSci Advisors
(617) 430-7576