CORMEDIX INC. REPORTS THIRD QUARTER 2020 FINANCIAL RESULTS AND PROVIDES BUSINESS UPDATE
Conference Call Scheduled for Today at 4:30 p.m. Eastern Time
Berkeley Heights, NJ – November 5, 2020 – CorMedix Inc. (NYSE American: CRMD), a biopharmaceutical company focused on developing and commercializing therapeutic products for the prevention and treatment of infectious and inflammatory disease, today announced financial results for the third quarter and nine months ended September 30, 2020 and provided an update on recent developments.
Recent Corporate and Regulatory Highlights:
- CorMedix continues its interactions with the FDA regarding the New Drug Application, or NDA, for Defencath™ for the prevention of catheter related blood stream infections, or CRBSIs, in patients undergoing hemodialysis via central venous catheter. The FDA has tentatively scheduled the previously announced meeting of the Antimicrobial Drugs Advisory Committee for January 14, 2021 to discuss the Defencath NDA.
- Recent changes to the CorMedix Board of Directors include the addition of Paulo Costa and Greg Duncan. These individuals further augment the expertise at the Board level on commercial leadership as well as corporate strategy. Each of Paulo and Greg has had successful careers and leadership roles in the biopharma industry.
- CorMedix has continued to expand its efforts to prepare for the commercial launch of Defencath. This includes ongoing dialogue with key payors and dialysis providers and ongoing market research. The interactions have been positive and clearly position CorMedix to ensure that, once Defencath is approved by the FDA, it will be in the best possible position to successfully launch in the US market.
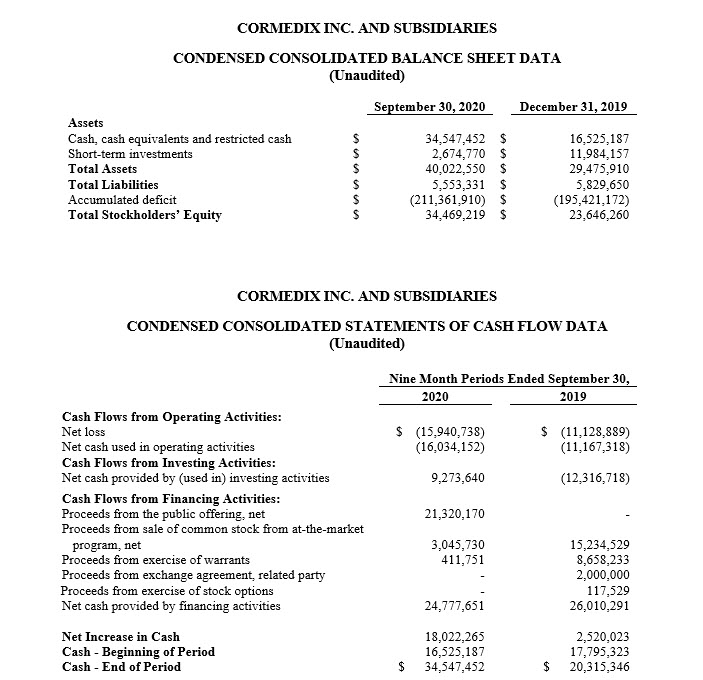
- Cash and short-term investments, excluding restricted cash, at September 30, 2020 amounted to $37.0 million. Pro forma cash, including cash on the balance sheet at September 30, 2020 and the net proceeds from recent ATM issuance, is approximately $41.8 million.
Khoso Baluch, CorMedix CEO, commented, “We have continued to make progress on our goal of bringing Defencath to the U.S. market as a catheter lock solution for hemodialysis. We look forward to discussing Defencath with the Antimicrobial Drugs Advisory Committee in January, ahead of the February 28, 2021 PDUFA date for the product. We also are making necessary preparations for the launch of Defencath in the U.S. hemodialysis market, following FDA approval. We believe we have the team, the focus, the resources, and a novel catheter lock solution that will meaningfully improve patient outcomes and are excited about the opportunities in front of us.”
Third Quarter and Nine Month 2020 Financial Highlights
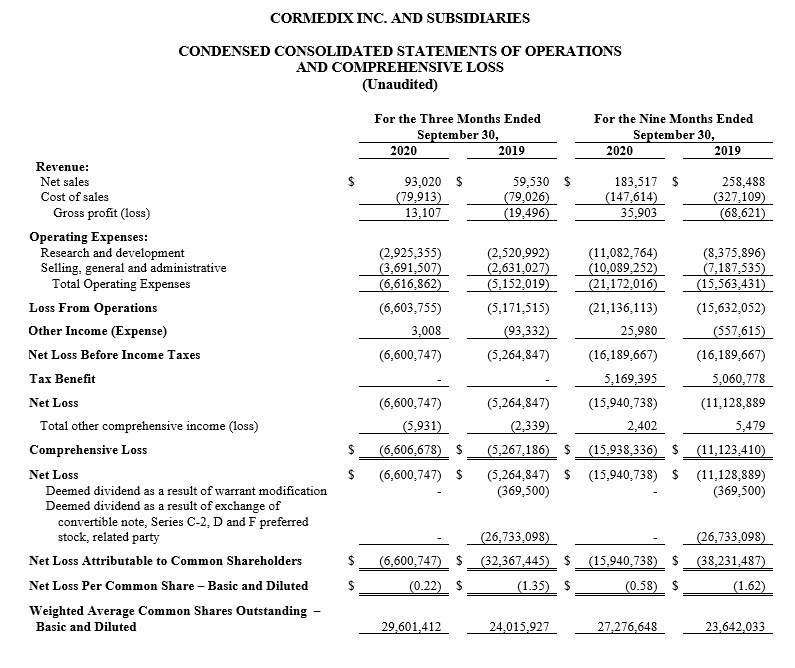
For the third quarter of 2020, CorMedix recorded a net loss attributable to common shareholders of $6.6 million, or $0.22 per share, compared with a net loss of $5.3 million, or $0.22 per share, in the third quarter of 2019, excluding the impact of deemed dividends recognized in September 2019 as the result of an exchange agreement and warrant modification.
For the nine months ended September 30, 2020, CorMedix recorded a net loss attributable to common shareholders of $15.9 million, or $0.58 per share, compared with a net loss of $11.1 million, or $0.47 per share, in the first nine months of 2019, excluding the impact of deemed dividends recognized in September 2019 as the result of an exchange agreement and warrant modification. The increase in net loss in the first nine months of 2020 was driven primarily by increased operating expenses.
Operating expenses during the third quarter of 2020 were $6.6 million, compared with $5.2 million in the third quarter of 2019, an increase of approximately 28%. This increase was due to a $0.4 million, or 16%, increase in R&D expense and $1.1 million, or 40%, increase in SG&A expense. Operating expenses during the nine-month period ended September 30, 2020 amounted to $21.2 million compared with $15.6 million during the comparable period in 2019, an increase of $5.6 million, or 36%, due to a 32% increase in R&D expense and 40% increase in SG&A for this period. R&D expense for the first nine months of 2020 included approximately $3.8 million in costs related to the purchase of raw materials and manufacturing of Defencath prior to its potential marketing approval and also included increased staffing costs. Higher SG&A costs were primarily driven by higher staffing costs as well as costs related to market research studies in preparation for the potential market approval of Defencath.
In July 2020, CorMedix completed an underwritten public offering of its common stock, which yielded net proceeds of approximately $21.3 million. The public offering was made pursuant to an underwriting agreement relating to the issuance and sale of an aggregate of 5,111,110 shares of common stock, including 666,666 shares of common stock pursuant to the full exercise of the underwriters’ option, at a public offering price of $4.50 per share. During October 2020, CorMedix issued shares under its at-the-market (“ATM”) program and realized net proceeds of approximately $4.6 million.
Total cash on hand and short-term investments as of September 30, 2020 amounted to $37.0 million, excluding restricted cash of $0.2 million. The Company believes that, based on the Company’s cash resources at September 30, 2020, and including the net proceeds received in October 2020 from ATM issuance, it has sufficient resources to fund operations for at least the coming 12 months, including the costs related to the initial preparations for the commercial launch of Defencath.
Conference Call Information
The management team of CorMedix will host a conference call and webcast today, November 5, 2020, at 4:30 PM Eastern Time, to discuss recent corporate developments and financial results. Call details and dial-in information is as follows:
Domestic: 800-917-9975
International: 212-231-2901
Conference ID: 21971218
Webcast: Webcast Link
About CorMedix
CorMedix Inc. is a biopharmaceutical company focused on developing and commercializing therapeutic products for the prevention and treatment of infectious and inflammatory diseases. The Company is focused on developing its lead product Defencath™, a novel, antibacterial and antifungal solution designed to prevent costly and life-threatening bloodstream infections associated with the use of central venous catheters in patients undergoing chronic hemodialysis. Defencath’s NDA has been filed and accepted for priority review with a PDUFA date of February 28, 2021. Defencath has been designated by FDA as Fast Track and as a Qualified Infectious Disease Product, which provides an additional five years of marketing exclusivity, which will be added to the five years granted to a New Chemical Entity upon approval of the NDA. CorMedix also intends to develop Defencath as a catheter lock solution for use in oncology and total parenteral nutrition patients. It is leveraging its taurolidine technology to develop a pipeline of antimicrobial medical devices, with programs in surgical sutures and meshes, and topical hydrogels. The Company is also working with top-tier researchers to develop taurolidine-based therapies for rare pediatric cancers. Neutrolin® is CE Marked and marketed in Europe and other territories as a medical device. For more information, visit: www.cormedix.com.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 that are subject to risks and uncertainties. All statements, other than statements of historical facts, regarding management’s expectations, beliefs, goals, plans or CorMedix’s prospects, future financial position, financing plans, future revenues and projected costs should be considered forward-looking. Readers are cautioned that actual results may differ materially from projections or estimates due to a variety of important factors, including: the results of our discussions with the FDA regarding the Defencath development path, including whether a second Phase 3 clinical trial for Defencath’s marketing approval will be required; the resources needed to secure approval of the new drug application for Defencath from the FDA; the risks and uncertainties associated with CorMedix’s ability to manage its limited cash resources and the impact on current, planned or future research, including the continued development of Defencath/Neutrolin and research for additional uses for taurolidine; obtaining additional financing to support CorMedix’s research and development and clinical activities and operations; preclinical results are not indicative of success in clinical trials and might not be replicated in any subsequent studies or trials; and the ability to retain and hire necessary personnel to staff our operations appropriately. At this time, we are unable to assess whether, and to what extent, the uncertainty surrounding the Coronavirus pandemic may impact our business and operations. These and other risks are described in greater detail in CorMedix’s filings with the SEC, copies of which are available free of charge at the SEC’s website at www.sec.gov or upon request from CorMedix. CorMedix may not actually achieve the goals or plans described in its forward-looking statements, and investors should not place undue reliance on these statements. CorMedix assumes no obligation and does not intend to update these forward-looking statements, except as required by law.
Investor Contact:
Dan Ferry
Managing Director
LifeSci Advisors
(617) 430-7576